Breaking In
A self-driving DNA delivery workcell for exploring biology.
TL;DR (bioRxiv)
DNA delivery—not editing—is the rate limiting step for studying and engineering organisms in our biosphere.
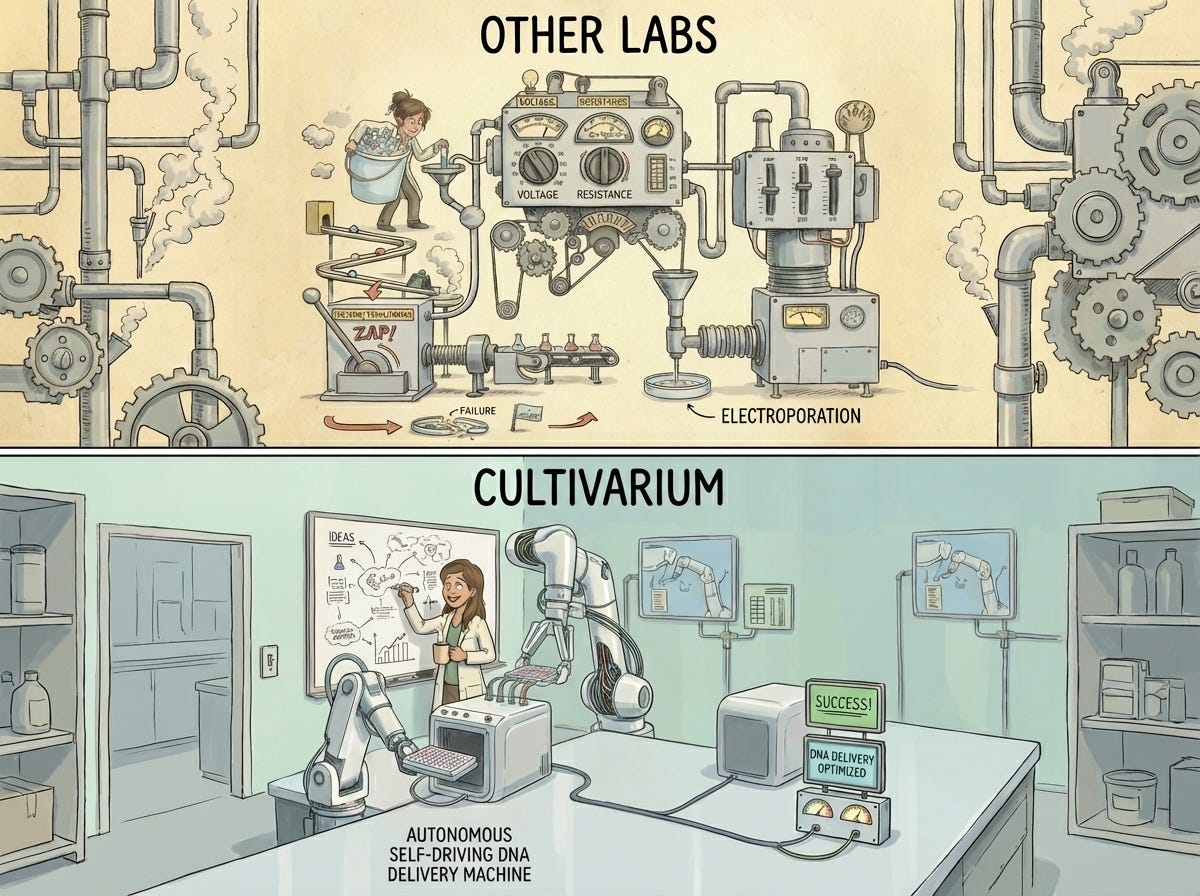
Existing electroporators are black-box tools built for E. coli, not for the thousands of microbes we already know how to culture.
We built a fully programmable, robotic, active-learning workcell to make DNA delivery systematically measurable so that we can collect data towards making it predictable.
This platform discovers electroporation conditions and functional plasmids simultaneously across diverse non-model organisms.
Using this system, we improved DNA delivery in Cupriavidus necator by 8.6× over the state of the art, in just three weeks.
It’s our first step toward a universal framework for entering any organism—and unlocking the rest of biology.
The story behind the paper
Exploring biology isn’t held back by a lack of imagination or ambition. It’s held back by a bottleneck that few dare to tackle - DNA delivery.
We talk a lot about accelerating biology—cheaper DNA synthesis, automated assays, large-scale sequencing, AI agents—but none of it matters if you can’t get DNA into the organism you want to study. You can culture thousands of microbes. You can design constructs, assemble pathways, even write whole genomes. But the fundamental questions about how an organism operates goes unanswered unless you can get synthetic DNA across the membrane. Without this, you can look but you can’t touch.
When we went searching for a framework—a systematic way to establish DNA delivery across species—we found almost nothing. Scientists often start with conjugation from E. coli and hope for the best. Other methods—chemical competence, natural competence, electroporation, biolistics—appear sporadically in the literature as organism-specific methods. There are no general rules, little quantitative data, and nothing close to a predictive model.
We wanted a method that could apply broadly, across as many microbes as possible. That led us to electroporation: a biophysical approach where an electrical pulse temporarily opens pores and DNA is electrophoresed into the cell… in theory. In practice, the field provides little in the way of biophysical understanding or guidance about which buffers, voltages, resistances, or capacitances matter.
If we wanted to learn about electroporation—even for one microbial strain—we needed many measurements. And to get those measurements, we needed instrumentation that didn’t exist. So we built it.
What began as a search for DNA delivery protocols became a multi‑year effort to design the assays, hardware, robotics, and machine‑learning infrastructure that now form the basis of a self‑driving laboratory workcell for discovering DNA delivery in non‑model microbes.
The Bottleneck We Couldn’t Ignore
DNA delivery is biology’s silent rate‑limiter. We can enumerate microbes using sequencing and we can synthesize pools of synthetic DNA, but without a reliable way to introduce DNA into a new organism, we remain observers rather than engineers. Phenomena remain descriptions rather than explanations.
Once we recognized this, we reframed the challenge: not “How do we electroporate this microbe?” but “How do we systematically learn to electroporate any microbe?”
That required three things:
A standardized, information‑rich assay.
Hardware capable of exposing, measuring, and varying the full electroporation parameter space.
A robotic and computational system capable of exploring that space efficiently.
None of these existed. So we built all three.
Building a Platform the Field Never Built
Modern commercial electroporators hide nearly everything that matters. They work well for E. coli, yeast, or mammalian cells—but what about everything else? Three knobs and a few numbered programs aren’t enough. There’s no way to programmatically control or systematically vary all the parameters for the electrical stimulus and to record the pulse.
We needed an instrument that behaved like a scientific tool, not a black box. That required custom high‑voltage architecture, including large capacitors reminiscent of 32‑oz craft beers—big enough to deliver precise, programmable pulses across a 96‑well plate. We needed a fully programmable system that could generate custom waveforms, control timing down to microseconds, and record the resulting traces.
Then we integrated this custom hardware with the rest of the laboratory: liquid handling, plating, incubation, and imaging. These are all controled by robotics powered by modern multimodal perception, Video‑Language Models (VLMs) and Vision‑Language‑Lab‑Action (VLLA) models, to interpret sensor data, press buttons on legacy instruments, and close the loop between instruction and action. Natural language became a control layer for scientists to do the experimentation they wanted. The system could explore, measure, and iterate—exactly what is required to explore biology.
An Initial Framework for Electroporation
We began by building a compact, informative 24‑condition screen—combinatorial variations of buffers, voltages, and waveforms—designed to provide the first measurable foothold for any new organism. This screen revealed electroporation conditions for bacteria that had no prior genetic tools.
But DNA delivery isn’t just about the pulse; it’s also about the plasmid. So we paired the electroporation screen with POSSUM, our pooled library of plasmids carrying diverse origins of replication and selection markers. With one experiment, we could determine both which electroporation conditions work and which plasmids replicate.
Finally, we incorporated active learning—a machine‑learning approach that lets the system choose conditions based on both exploration and knowledge extracted from the literature.
Using this self‑driving DNA delivery system, we improved electroporation for Cupriavidus necator by 8.6× over the published protocol—first described 31 years ago—in just three weeks. The updated method is available in our preprint for researchers working on bioplastics, carbon capture, valorization, and bioremediation.
Getting to a Predictive Model of DNA Delivery
Electroporation was our proving ground—the clearest illustration of what happens when a field lacks measurement, models, and systematic tools. DNA delivery remained artisanal not because it was unknowable, but because no one had built the instrumentation required to know it.
Now that we’ve built the hardware, robotics, and active‑learning pipeline for self‑driving experimentation, we can collect the kind of data required to learn DNA delivery at scale.
This is our long‑term goal: a predictive framework for DNA delivery across the entire tree of life. A way to transform genetic tractability from a bespoke art into a discoverable property. A way to reliably unlock organisms with extraordinary abilities—those that remediate toxins, fix carbon in extreme chemistries, regenerate tissues, metabolize plastics, tolerate vacuum or radiation, and survive desiccation for years.
Our system can already execute multiple Cultivarium assays end‑to‑end, and electroporation is now part of that unified framework. We will grow this platform into a self‑driving genetic‑tractability workcell—one designed not merely to run experiments, but to learn from them.
The Road Ahead
The deeper story is that this entire system—instrumentation, robotics, multimodal perception, and active learning—is the foundation of a self‑driving genetic‑tractability workcell. A modular unit capable of executing Cultivarium‑scale assays with minimal human intervention and full scientific intent.
Over the next year, we’ll begin opening access to this platform as a Cultivarium‑operated cloud lab. Scientists will be able to submit organisms, constructs, or hypotheses and receive data, protocols, and actionable insights back—without needing specialized automation expertise. Tools that help us learn biology faster should help everyone learn biology faster.
Electroporation was our test case—an area where assumptions were weak, models nonexistent, and systematic measurement missing. It showed that once the right instrumentation exists, biology becomes legible at resolutions and throughput that human‑only experimentation and traditional literature have not reached.
Now imagine applying this same philosophy—frameworks, measurement, automation, and predictive modeling—to every major bottleneck in studying and engineering new organisms.
That’s the next frontier for Cultivarium: a world where genetic tractability is discoverable, DNA delivery is predictable, and the full diversity of biology is accessible to science and engineering.


Very cool! I spent an embarrassingly long time during my PhD trying to optimise electroporations in mouse and (separately) human T cells. Nucleic acid/protein delivery is a huge unmet need. I’ll read the pre-print this weekend!